- 2,606
- 2,354
- 113
- Location
- Efland, NC
The initial cooldown of the jugs comes out to 138 btu/hr. Very much in line with the first test.
Steel Soldiers now has a few new forums, read more about it at: New Munitions Forums!

Sounds like your system is not running properly. Could be those wires coming out of the cooler box letting in warm air. Like I mentioned earlier, mine froze 2 gallons of water in under 6 hours.The initial cooldown of the jugs comes out to 138 btu/hr. Very much in line with the first test.
Certainly a possibility. The wires are very fine so I would expect the leakage would be minimal.Sounds like your system is not running properly. Could be those wires coming out of the cooler box letting in warm air. Like I mentioned earlier, mine froze 2 gallons of water in under 6 hours.
Looking at the datasheet for the compressor and the MAX capacity is well within the range of what would work for a coolshirt refrigerator.Hi
How about using one of these (set to cooler not to freezer) and the Cool Shirts they use in racing? Or would the thermal load from cooling a person be more than they could handle?
Cheers Phil
So like I said all along, it is as capable as any house freezer. Yes it "can" freeze something put in it. It doesn't have to be frozen first.I managed to snap a photo of the label on the compressor. It is a Danoss 101Z0200.
The attached datasheet for the compressor indicates the capacity of the compressor. The values I've recorded are within the reasonable range of what you would expect for this unit.
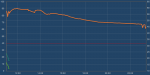
Well I can test my "2 gallons" of water again (16 Ibs) but I'm sure the results will be the same again. I'll start tomorrow.Sure it can freeze something. It will take a good bit longer to do so than a home freezer will based on the tests of the two units I have. I stand by my original statement that is isn't well suited for dumping a bunch of stuff inside to freeze. It is better suited to keep things already frozen in that state.
To eliminate the probes as cause for the freezing times I reset and used freezer bags with water in place of the milk jugs. They have a little better geometry in regards to volume/surface area ratio.
I had a total of 8 lbs of water that started off at 46 degrees F. The water took approximately 11 hours to cool and freeze solid. The time is based on watching the condenser discharge temperature to detect when the compressor started cycling again after the heat load was placed in the chamber.
If you calculate it out it comes out to about 110 btu/hr average over that time. That is the same basic result as the test with the milk jugs.
So maybe the units I have are down on capacity or their insulation is degraded. Totally possible. The seals are in very good condition as they had been recently serviced per the documentation.
View attachment 721588
Hi,
After all all of these posts I am now brain washed into wanting one (it's like an advert, if you see it often enough you want one!)
who has them them for the best price in the US or as you guys in the US say, who has the best deal?
thanks Mark
